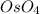Mr[K2SO4] = Ar[K] * 2 + Ar[S] + Ar[O] * 4 = 39.0983 * 2 + 32.065 + 15.9994 * 4 = 174.2592
Mr[LiOH] = Ar[Li] + Ar[O] + Ar[H] = 6.941 + 15.9994 + 1.00794 = 23.94834
Mr[CuNO3] = Ar[Cu] + Ar[N] + Ar[O] * 3 = 63.546 + 14.0067 + 15.9994 * 3 = 125.5509
Mr[CaBr2] = Ar[Ca] + Ar[Br] * 2 = 40.078 + 79.904 * 2 = 199.886
Mr[MgCO3] = Ar[Mg] + Ar[C] + Ar[O] * 3 = 24.305 + 12.0107 + 15.9994 * 3 = 84.3139
Mr[CaSO3] = Ar[Ca] + Ar[S] + Ar[O] * 3 = 40.078 + 32.065 + 15.9994 * 3 = 120.1412
Mr[CaCl2] = Ar[Ca] + Ar[Cl] * 2 = 40.078 + 35.453 * 2 = 110.984
Mr[BaSO4] = Ar[Ba] + Ar[S] + Ar[O] * 4 = 137.327 + 32.065 + 15.9994 * 4 = 233.3896
Mr[H3PO4] = Ar[H] * 3 + Ar[P] + Ar[O] * 4 = 1.00794 * 3 + 30.973762 + 15.9994 * 4 = 97.995182
Объяснение:

Номер 2.*Выбирай, что нравится. Все наименования, написанные первыми - систематические, т.е. используются на "официальном" уровне, среди химиков, международная - лично я писал бы их.
Номер 3.а)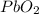
б)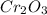
в)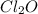
г)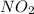
д)