Https://minhag.ucsc.edu/node/6768
https://minhag.ucsc.edu/node/6769
https://minhag.ucsc.edu/node/6771
https://minhag.ucsc.edu/node/6772
https://minhag.ucsc.edu/node/6773
https://minhag.ucsc.edu/node/6774
https://minhag.ucsc.edu/node/6713
https://minhag.ucsc.edu/node/6720
https://minhag.ucsc.edu/node/6716
https://minhag.ucsc.edu/node/6714
https://minhag.ucsc.edu/node/6730
https://minhag.ucsc.edu/node/6718
https://minhag.ucsc.edu/node/6717
https://minhag.ucsc.edu/node/6759
https://minhag.ucsc.edu/node/6760
https://minhag.ucsc.edu/node/6454
https://minhag.ucsc.edu/node/6455
https://minhag.ucsc.edu/node/6761
https://minhag.ucsc.edu/node/6762
https://minhag.ucsc.edu/node/6763
https://minhag.ucsc.edu/node/6765
https://minhag.ucsc.edu/node/6766
https://minhag.ucsc.edu/node/6767
https://minhag.ucsc.edu/node/6456
https://minhag.ucsc.edu/node/6457
https://minhag.ucsc.edu/node/6458
https://minhag.ucsc.edu/node/6460
https://minhag.ucsc.edu/node/6459
https://minhag.ucsc.edu/node/6463
https://minhag.ucsc.edu/node/6461
https://minhag.ucsc.edu/node/6462
https://minhag.ucsc.edu/node/6465
https://minhag.ucsc.edu/node/6464
https://minhag.ucsc.edu/node/6594
https://minhag.ucsc.edu/node/6596
https://minhag.ucsc.edu/node/6597
https://minhag.ucsc.edu/node/6595
https://minhag.ucsc.edu/node/6598
https://minhag.ucsc.edu/node/6599
https://minhag.ucsc.edu/node/6600
https://minhag.ucsc.edu/node/6601
https://minhag.ucsc.edu/node/6602
https://minhag.ucsc.edu/node/6603
https://minhag.ucsc.edu/node/6604
https://minhag.ucsc.edu/node/6613
https://minhag.ucsc.edu/node/6605
https://minhag.ucsc.edu/node/6606
https://minhag.ucsc.edu/node/6607
https://minhag.ucsc.edu/node/6608
https://minhag.ucsc.edu/node/6609
https://minhag.ucsc.edu/node/6610
https://minhag.ucsc.edu/node/6611
https://minhag.ucsc.edu/node/6612
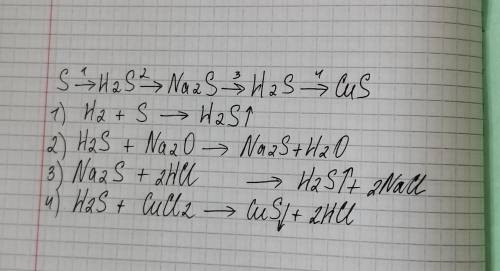
14.8 г
Объяснение:
Уравнение реакции:
CH3-COOH + CH3-OH <=> CH3-COO-CH3 + H2O ( метиловый эфир уксусной кислоты)
Находим молярные массы:
М(CH3COOH) = 12+1*3+12+16*2+1 = 60 г/моль
М(CH3OH) = 12+1*3+16+1 = 32г/моль
M(CH3-COO-CH3) = 12+1*3+12+16*2+12+1*3 = 74 г/моль
Смотрим сколько вещества прореагировало по факту:
n(CH3COOH) = 24:60 =0.4 моль
n(CH3OH) = 6.4:32 = 0.2 моль
В избытке - кислота, в недостатке - спирт, значит в результате реакции не может получиться больше чем 0.2 моль эфира.
В 1 моль метилацетата 74 г вещества, значит в 0.2 моль 74*0.2 = 14.8 г.